Published manuscript describing regional variation in vasular and cardiac myocyte properties in the cardiac SAN
We published a new manuscript in collaboration with the Santana lab. We are super excited for this collaboration and it is our first manuscript with work done at UC Davis.
Grainger, N, Guarina, L, Cudmore, RH, and Santana, LF (2021). The Organization of the Sinoatrial Node Microvasculature Varies Regionally to Match Local Myocyte Excitability. Function (Oxf) 2, zqab031. doi:10.1093/function/zqab031.
For an excellent overview, please also see the Perspectives by Scott Early (University of Nevada at Reno) and Jonathan Lederer (University of Maryland at Baltimore).
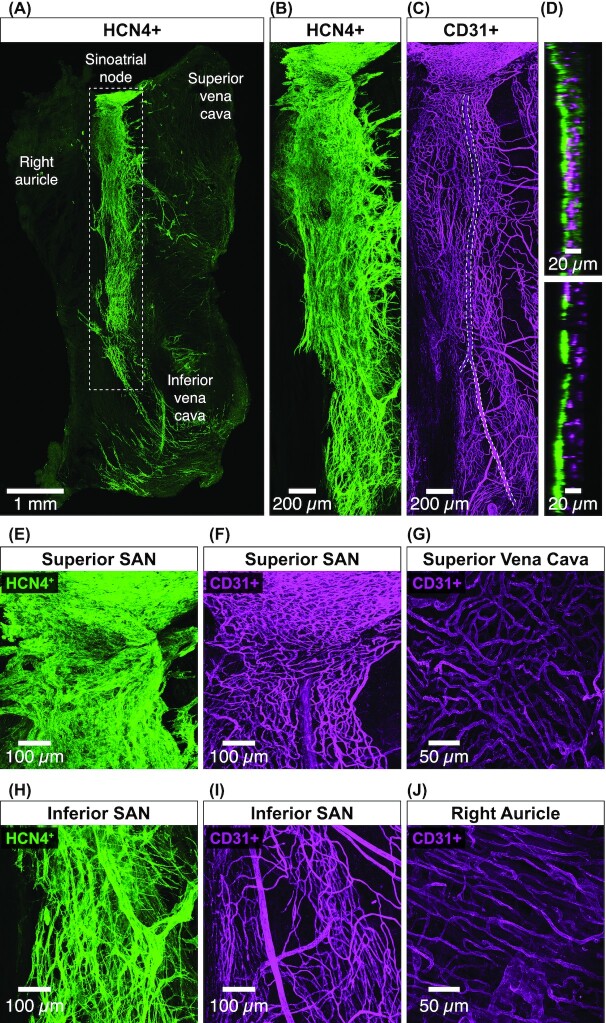
The cardiac cycle starts when an action potential is produced by pacemaking cells in the sinoatrial node. This cycle is repeated approximately 100 000 times in humans and 1 million times in mice per day, imposing a monumental metabolic demand on the heart, requiring efficient blood supply via the coronary vasculature to maintain cardiac function. Although the ventricular coronary circulation has been extensively studied, the relationship between vascularization and cellular pacemaking modalities in the sinoatrial node is poorly understood. Here, we tested the hypothesis that the organization of the sinoatrial node microvasculature varies regionally, reflecting local myocyte firing properties. We show that vessel densities are higher in the superior versus inferior sinoatrial node. Accordingly, sinoatrial node myocytes are closer to vessels in the superior versus inferior regions. Superior and inferior sinoatrial node myocytes produce stochastic subthreshold voltage fluctuations and action potentials. However, the intrinsic action potential firing rate of sinoatrial node myocytes is higher in the superior versus inferior node. Our data support a model in which the microvascular densities vary regionally within the sinoatrial node to match the electrical and Ca2+ dynamics of nearby myocytes, effectively determining the dominant pacemaking site within the node. In this model, the high vascular density in the superior sinoatrial node places myocytes with metabolically demanding, high-frequency action potentials near vessels. The lower vascularization and electrical activity of inferior sinoatrial node myocytes could limit these cells to function to support sinoatrial node periodicity with sporadic voltage fluctuations via a stochastic resonance mechanism.